CBD and the Law
From Prohibition to the Farm Bill to the Regulatory Future
-

By Jeremy Amos
Published: Monday, Oct 6, 2025
The story of CBD is, in many ways, the story of law and regulation. Unlike other wellness ingredients that quietly entered the marketplace, CBD has been bound up in legal battles, political debates, and regulatory gray zones from the very beginning.
For decades, prohibition kept hemp and marijuana under the same legal umbrella, stifling innovation and limiting scientific research. When the 2018 Farm Bill removed hemp from the Controlled Substances Act, the CBD market exploded almost overnight. But while cultivation became legal, the rules governing products, labeling, and sales remained confusing.
Today, we find ourselves at a crossroads: consumer demand is stronger than ever, science is catching up, but regulators are still struggling to create a consistent framework. Understanding CBD’s legal journey - yesterday’s prohibitions, today’s patchwork, tomorrow’s opportunities - is critical for any brand that wants to succeed long-term.
Yesterday: Prohibition and Restriction
For most of the 20th century, cannabis in all forms - marijuana and hemp alike—was treated as one thing: a prohibited drug.
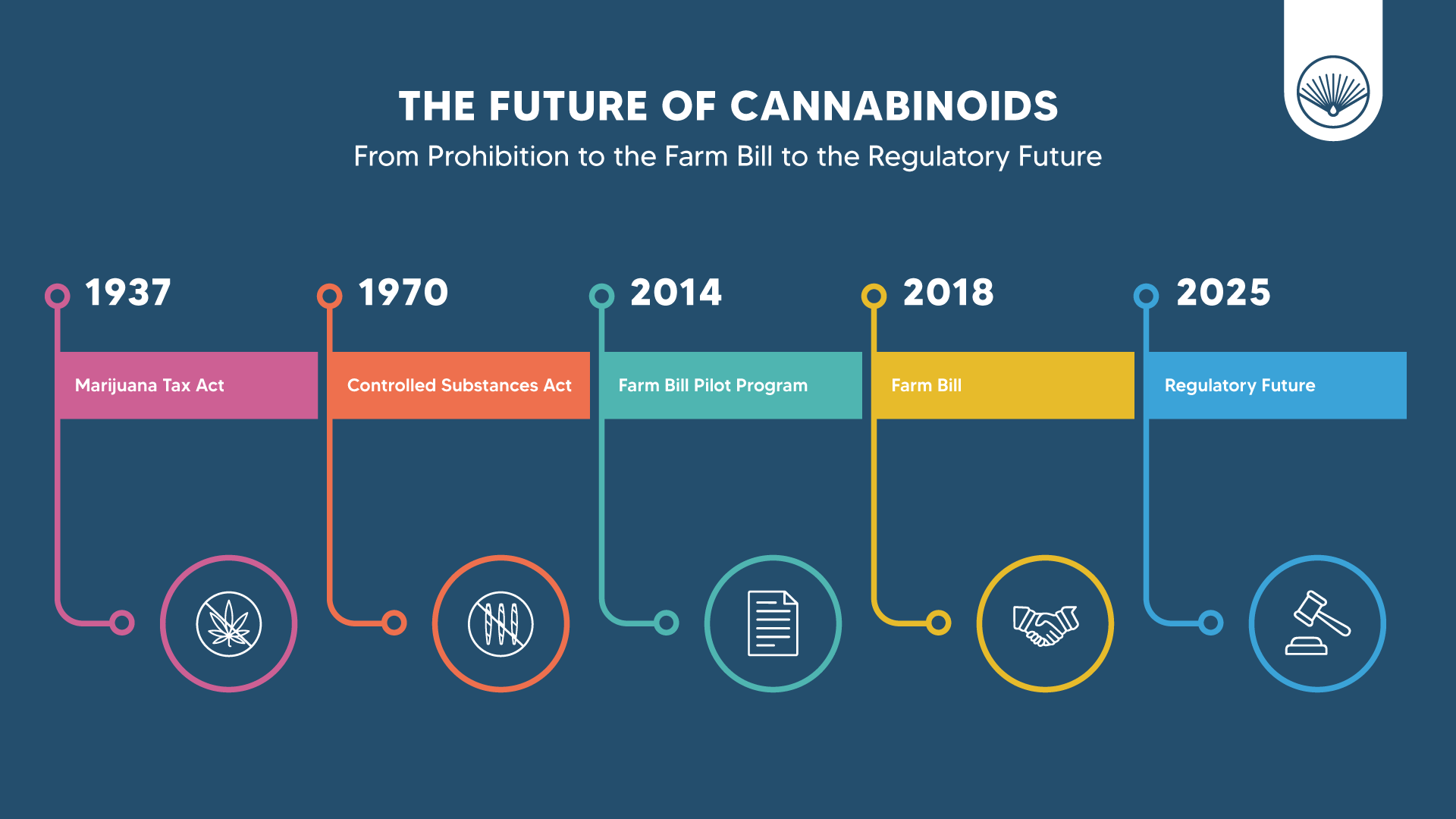
- 1937 Marijuana Tax Act - effectively criminalized all forms of cannabis cultivation and sale, even hemp grown for industrial use.
- 1970 Controlled Substances Act (CSA) - formally classified cannabis as a Schedule I drug, defined as having “no accepted medical use and a high potential for abuse.” This designation lumped hemp and marijuana together, despite hemp containing negligible levels of THC.
- Research restrictions - any studies involving cannabinoids, including CBD, required special federal approval, making clinical research rare and slow.
Takeaway: Yesterday’s laws erased the distinction between hemp and marijuana, creating barriers that delayed CBD research and commercialization for decades.
Today: The Farm Bill Era
The past decade has been transformative.
- 2014 Farm Bill pilot program - allowed research institutions and state departments of agriculture to grow hemp under limited conditions.
- 2018 Farm Bill - fully legalized hemp (defined as cannabis with ≤0.3% Δ9-THC), removed it from the CSA, and allowed for large-scale cultivation and sale.
This legislation created the modern hemp-derived CBD industry. But legalization didn’t bring clarity everywhere.
FDA’s stance
The FDA maintains that CBD cannot be marketed as a dietary supplement or added to foods under existing regulatory frameworks. That means gummies, beverages, and snacks with CBD sit in a gray area.
The State Patchwork
Because federal law left gaps, each state created its own rules. Some allow CBD in foods and beverages, others restrict or prohibit it. This patchwork creates compliance headaches for brands trying to distribute nationally.
The State Patchwork
Innovations like D8 THC and D10 THC - psychoactive cannabinoids synthesized from hemp-derived CBD - have further complicated the landscape. Some states have banned them outright, others allow them, and Congress is now weighing 2025 proposals that could restrict intoxicating hemp derivatives altogether.
Takeaway: Today’s legal framework is a mix of progress and patchwork - hemp-derived CBD is legal, but the rules vary dramatically depending on product type and geography.
Tomorrow: Toward Clarity
The legal future of CBD is still unwritten, but several trends are clear.
A New Federal Pathway
In 2023, the FDA formally asked Congress to create a new regulatory pathway for CBD. This would likely include:
- Serving size and daily dosage limits.
- Labeling requirements (warnings, disclaimers, batch testing).
- Age restrictions and packaging guidelines.
If enacted, this would bring CBD products into line with established categories like dietary supplements and functional foods—unlocking mainstream retail and consumer trust.
Global harmonization
The U.S. isn’t alone in wrestling with CBD. The EU’s Novel Foods regulation governs CBD edibles, while countries like Australia and Japan have tightly controlled frameworks. Over time, international standards will converge, especially as CBD enters global supply chains.
Cannabis convergence
As broader cannabis reform progresses, CBD will increasingly be regulated in tandem with THC and other cannabinoids. Expect future laws to treat “cannabinoids” as a class, with tiered rules for intoxicating vs. non-intoxicating compounds.
Takeaway: Tomorrow, CBD law will be less about if it’s legal, and more about how it’s standardized and regulated across borders.
What This Means for Brands (and OBX)
For brands, the message is simple: prepare today for tomorrow’s laws.
- Stay audit-ready. Operating under cGMP, NSF, and ISO standards now will smooth compliance later.
- Invest in labeling and transparency. QR codes linked to full-panel COAs are already becoming industry standard.
- Think global. Design products with international compliance in mind to avoid reformulation later.
At OBX, we take a proactive stance: if a product can’t stand up to tomorrow’s regulations, we won’t make it today.